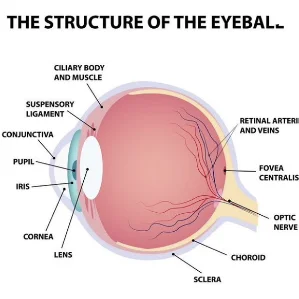
Retina Specialty Institute is a renowned ophthalmology practice that proudly serves patients across Northwest Florida, Central Florida, Mississippi, and the Alabama Gulf Coast. Our esteemed, skilled eye doctors provide transformative retina care in all aspects of service—from personalized treatments to advanced techniques to state-of-the-art surgical procedures. RSI is the trusted partner of countless people across the US looking to protect and enhance their vision.
As part of our commitment to continued advancement and innovation in the ophthalmology field and eye/retina treatment, our practice offers clinical trials on an ongoing basis. Learn more about our current clinical trials below and contact us now for any questions or to book an appointment with a physician.
4D-150-C003 (named 4Front-1)
A Phase 3 Randomized, Double-Masked, Active-Controlled Trial of a Single Intravitreal Injection of 4D-150 in Adults with Macular Neovascularization Secondary to Age-Related Macular Degeneration (4FRONT-1)
Sponsor: 4D Molecular Therapeutics, Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Open to enrollment
APL2-GA-411 (Garland)
A Prospective, Multicenter, Open-Label, Observational Phase 4 Study to Evaluate Real-World Safety, Tolerability, and Treatment Patterns of Pegcetacoplan (Syfovre) in Patients With Geographic Atrophy Secondary to Age-Related Macular Degeneration
Sponsor: Apellis Pharmaceuticals
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Open to enrollment
ML43000
A Phase IV, Multicenter, Open-Label, Single-Arm Study Of The Response To Treatment After Transition To The Port Delivery System With Ranibizumab (Susvimo™ [Ranibizumab Injection]) In Patients With Neovascular Age-Related Macular Degeneration Previously Treated With Intravitreal Agents Other Than Ranibizumab
Sponsor: Genentech, Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Open to enrollment
Burgundy (BP41670)
A Three-Part, Phase I Study to Investigate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Zifibancimig Following Intravitreal Administration of Multiple Ascending Doses and Continuous Delivery from the Port Delivery in Patients with Neovascular Age-Related Macular Degeneration
Sponsor: Hoffmann-La Roche
PI: Sunil Gupta, MD
Clinicaltrials.gov
Status: Open to enrollment
RTE888-E001
Neovascular (wet) Age-Related Macular Degeneration (NOVA-1) study – Phase 2; To evaluate the effect and durability of AR-14034 SR (340 μg and 680 μg) on best-corrected visual acuity (BCVA) compared with intravitreal standard of care anti-vascular endothelial growth factor (anti-VEGF) treatment in subjects with nAMD
Sponsor: Alcon/Aerie
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Open to enrollment
OTX-TKI-2023-AMD-301 (SOL)
A Phase 3, Multicenter, Double-Masked, Randomized, ParallelGroup Study to Evaluate the Efficacy and Safety of Intravitreal OTX-TKI (axitinib implant) in Subjects with Neovascular AgeRelated Macular Degeneration
Sponsor: Ocular Therapeutix, Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Enrollment is closed
OTX-TKI-2023-AMD-303 (SOL-R)
A Phase 3, Multicenter, Double-Masked, Randomized, Parallel Group Study to Evaluate the Efficacy and Safety of Intravitreal OTX-TKI (Axitinib Implant) in Subjects with Neovascular AgeRelated Macular Degeneration
Sponsor: Ocular Therapeutix, Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Closed to enrollment
EYP-1901-301 (Lugano)
A Phase 3, Multicenter, Prospective, Randomized, Double-Masked, Parallel-Group Study of EYP-1901, a Tyrosine Kinase Inhibitor (TKI), Compared to Aflibercept in Subjects with Wet AMD
Sponsor: EyePoint Pharmaceuticals, Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Closed to enrollment
QA102-CS201
A Phase 2, Double-Masked, Randomized, Placebo-Controlled, Dose-Response Study Assessing The Safety And Efficacy Of Qa102 In Subjects With Dry Age-Related Macular Degeneration (Amd)
Sponsor: Smilebiotek Zhuhai Limited
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Active – Enrollment is closed
VGFTe-HD-OD-2444
A Phase 3b Single-Arm Study of Aflibercept 8 mg in Participants with Neovascular Age-Related Macular Degeneration (nAMD) or Diabetic Macular Edema (DME)
Sponsor: Elara
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Active – Enrollment is closed
AVD-104-C01
A Single and Multiple Dose Study to Evaluate the Safety, Pharmacokinetics, and Treatment Effect of Intravitreal AVD-104 in Participants with Geographic Atrophy Secondary to Age-related Macular Degeneration
Sponsor: Siglec/Aviceda
Principal Investigator: John Myers, MD
Clinicaltrials.gov
Status: Active – Enrollment is closed
MR41927
Real-World, Long-Term Data Collection To Gain Clinical Insights Into Roche Ophthalmology Products (VOYAGER STUDY)
Sponsor: Hoffmann-La Roche
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Enrollment is closed
GR40549
A Multicenter, Open-Label Extension Study To Evaluate The Long-Term Safety And Tolerability Of The Port Delivery System With Ranibizumab In Patients With Neovascular Age-Related Macular Degeneration (PORTAL)
Sponsor: Hoffmann-La Roche
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Active – Enrollment is closed
OPH2005
A Phase 2b Randomized, Double-masked, Controlled Trial to Establish the Safety and Efficacy of Zimura™ (Complement C5 Inhibitor) Compared to Sham in Subjects with Autosomal Recessive Stargardt Disease
Sponsor: IVERIC bio, Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Active – Enrollment is closed
GR41675
A Phase III, Multicenter, Randomized Study Of The Efficacy, Safety, And Pharmacokinetics Of The Port Delivery System With Ranibizumab In Patients With Diabetic Retinopathy (PAVILION)
Sponsor: Nanoscope Therapeutics Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Active – Enrollment Closed
GR40550
A Phase III, Multicenter, Randomized, Visual Assessor-Masked, Active-Comparator Study Of The Efficacy, Safety, And Pharmacokinetics Of The Port Delivery System With Ranibizumab In Patients With Diabetic Macular Edema
Sponsor: Hoffmann-La Roche
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Active – Enrollment is Closed
VGFTe-HD-OD-2444
A Phase 3b Single-Arm Study of Aflibercept 8 mg in Participants with Neovascular Age-Related Macular Degeneration (nAMD) or Diabetic Macular Edema (DME)
Sponsor: Elara
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Active – Enrollment is closed
Retina Specialty Institute is currently not participating in any Retinal Vein Occlusion clinical trials at this time. Please check back or contact us to see if you are a candidate for any open trials.
Retina Specialty Institute is currently not participating in any Retinal Detachments clinical trials at this time. Please check back or contact us to see if you are a candidate for any open trials.
7317—CL-0003
A Phase 1b, Multicenter, Dose Escalation, Evaluation of Safety and Tolerability of ASP7317 for Geographic Atrophy Secondary to Age-related Macular Degeneration.
Sponsor: Astellas Institute for Regenerative Medicine
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Open to enrollment
ADVM-022-12
A Multi-Center, Randomized, Double-Masked, Active-Comparator-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of Ixoberogene soroparvovec (Ixo-vec) in Participants with Neovascular Age-Related Macular Degeneration (ARTEMIS)
Sponsor: Adverum Biotechnologies, Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Open to enrollment
81201887MDG2001
A Phase 2b, Randomized, Double-masked, Multicenter, Dose-ranging, Sham-controlled Clinical Trial to Evaluate Intravitreal JNJ-81201887 (AAVCAGsCD59) Compared to Sham Procedure for the Treatment of Geographic Atrophy (GA) Secondary to Age-related Macular Degeneration (AMD)
Sponsor: Janssen Research & Development, LLC
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Active – Enrollment is closed
RGX-314-2104
A Randomized, Partially Masked, Controlled, Phase 2b/3 Clinical Study to Evaluate the Efficacy and Safety of RGX-314 Gene Therapy in Participants with nAMD (ATMOSPHERE)
Sponsor: REGENXBIO Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Open to enrollment
RGX-314-3101
A Randomized, Partially Masked, Controlled, Phase 3 Clinical Study To Evaluate The Efficacy And Safety Of Rgx-314 Gene Therapy In Participants With nAMD
Sponsor: REGENXBIO Inc.
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Open to enrollment
4D-15-C001
A Phase 1/2 Dose-Escalation and Randomized, Controlled, Masked Expansion Trial of Intravitreal 4D-150 Gene Therapy in Adults with Neovascular (Wet) Age-Related Macular Degeneration
Sponsor: 4D Molecular Therapeutics
Principal Investigator: Sunil Gupta, MD
Clinicaltrials.gov
Status: Enrollment is closed
Clinical Trials FAQ
What is a clinical trial?
What are the benefits of participating in a clinical trial?
Reasons a patient may partake in a clinical trial may include:
- Potential benefits from a new drug or treatment
- Better methods for managing their symptoms
- Contributing to better future care for others
What clinical trials or enrolling studies are available at Retina Specialty Institute?
We engage in clinical trials that we feel will best advance the health needs of our patients, with special interest in those striving to impact leading causes of blindness:
- Age-Related Wet Macular Degeneration (Wet AMD)
- Diabetic Macular Edema/Diabetic Retinopathy (DME)
- Retinal Vein Occlusion (BRVO/CRVO)
How are clinical trials conducted?
Each clinical trial has a number of steps, which are known as phases. Each phase focuses on answering a specific research question.
- Phase I: This first phase of a clinical trial involves only a small group of people. During Phase I, researchers are focused on the safety of a drug or treatment.
- Phase II: Phase II of a clinical trial is also focused on safety, but this time the drug or treatment is given to a larger group of people.
- Phase III: In Phase III of a clinical trial, research is conducted with even larger groups of people. This part of the trial focuses on the effectiveness of a drug or treatment while monitoring side effects. At this time, the drug or treatment is also compared to commonly used treatments and additional information is collected to allow for safe use of the drug or treatment.
- Phase IV: Phase IV is conducted after a drug or treatment is already on the market. At this time, additional information can be gathered regarding how the drug affects a broader patient population and long-term side effects are evaluated.
Are all clinical trials effective?
Clinical trials provide patients either a promising new treatment or the best available conventional treatment. It is impossible to guarantee the success of a treatment. However, clinical trials have been proven to offer some of the most advanced retinal treatments available today.
Contact Retina Specialty Institute
To learn more about clinical trials, including whether you are a good candidate for an ongoing clinical trial, please complete the form below to join our research registry. You can also contact our Study Coordinator, Sarah Faruzzi, directly at sfaruzzi@retinaspecialty.com or call (850) 476-6759 x 940 with any clinical research related questions.
The doctors at Retina Specialty Institute have either authored or reviewed and approved this content.
Page Updated: